Creative Diagnostics of New York, has abruptly terminated the low cost testing service for Glyphosate in human body fluids and food.
This has resulted in disappointment on one side, and determination on the other, to continue search for a suitable lab that will provide low cost service for the people, at a pounce point that is within reach of average Canadians and Americans. This effort, including possibility of setting up a few “citizen’s labs” are on the drawing board. It is not certain when such labs may be available for the masses. We hope it will be sooner rather than later.
Meanwhile, here is an option still available to the people – albeit for a hundred USD per test, which might or might not include shipping charges.
Microbe Inotech Laboratories, 11754 Westline Industrial Drive, St. Louis, MO 63146, USA, (800) 688-9144
Breast milk (10+ samples) – $115/sample, minimum of 2mL
Water/urine (10+ samples) – $100/sample, minimum of 10mL
If you are able to obtain your own sampling containers (polypropylene bottles/vials), then there will be no additional charge. All samples should be shipped in suitable packaging to prevent the samples from being crushed in transit. In addition, the samples should be ‘cold-packed’ and/or frozen and shipping be expedited (1-2 day) if possible. Please let me know if you have any other questions. We look forward to working with you. Thanks.
Ben Winkler
Research Laboratory Manager
Microbe Inotech Laboratories, Inc.
(314) 645-2177 x104
Although the quotation of a hundred dollars should be valid for water and urine for a minimum order of 10 samples, he is willing to forego that for now, since Canadians need to test the waters first, especially if there is any glitch in the shipping of Human Specimens for lab test across the border, with the US Customs.
I have decided to try this out by sending my own urine etc for testing, via Fedex, and see what happens. There are some regulations to be followed, in order to ensure the shipment reaches the lab without getting stuck at customs. Correct packaging and labelling seems to be the key.
This blog post is made specifically to help with the people, in arranging their own shipment of specimens, to Microbe Inotech or any other lab in the US or other countries.
I have borrowed from the Fedex document called “Packaging Guideline for Clinical Samples”
It says:
Requirements for Clinical Samples
This guide outlines the requirements for shipping with FedEx Express. In addition, all shipments must comply with all applicable local, state and federal laws governing packing, marking and labeling. Blood, urine, fluids, and other specimens containing or suspected of containing infectious substances must be shipped according to applicable government, International Air Transport Association (IATA) and International Civil Aviation Organization (ICAO) regulations.
 For the purposes of this guide, clinical samples are generally defined as non-infectious human or animal materials including, but not limited to, excreta, secreta, tissue and tissue fluids, blood and FDA-approved pharmaceuticals that are blood products.
For the purposes of this guide, clinical samples are generally defined as non-infectious human or animal materials including, but not limited to, excreta, secreta, tissue and tissue fluids, blood and FDA-approved pharmaceuticals that are blood products.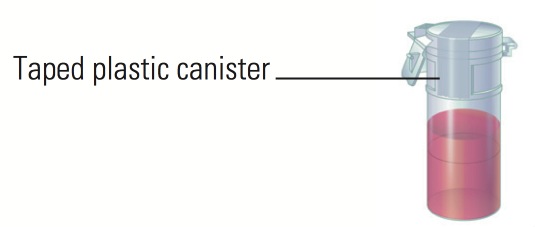
General Packaging Requirements
For liquid clinical samples, you must include four layers of packaging:
- Primary watertight inner receptacle. Use watertight containers for liquid specimens with:
- positive closure such as a screw-on, snap-on or push-on lid, taped for an additional seal. If you place multiple fragile primary receptacles in
- single secondary receptacle, they must be individually wrapped or separated to prevent contact between them.
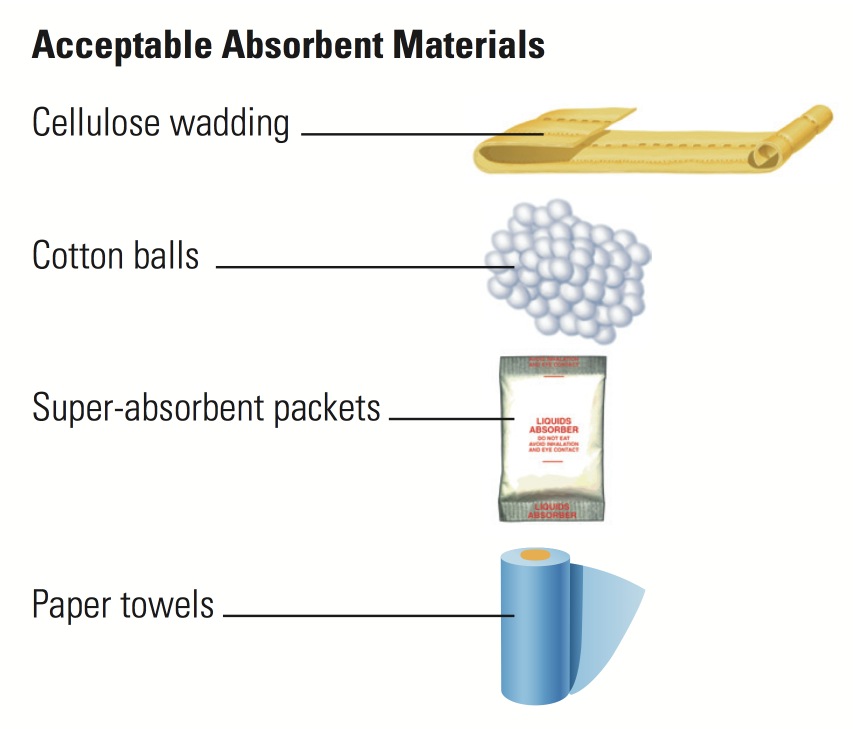
- Absorbent material. Place absorbent material between the primary and secondary receptacles, using enough material to absorb the entire contents of all primary receptacles. Acceptable absorbent materials include cellulose wadding, cotton balls, super-absorbent packets and paper towels.
- Secondary watertight inner receptacle. Use a watertight sealed plastic bag, plastic canister or screw-cap can.
- Sturdy outer packaging. Use rigid outer packaging constructed of corrugated fiberboard, wood, metal or plastic, appropriately sized for the contents. Chipboard or paperboard boxes are unacceptable outer packaging.


Black plastic box as sturdy outer packaging

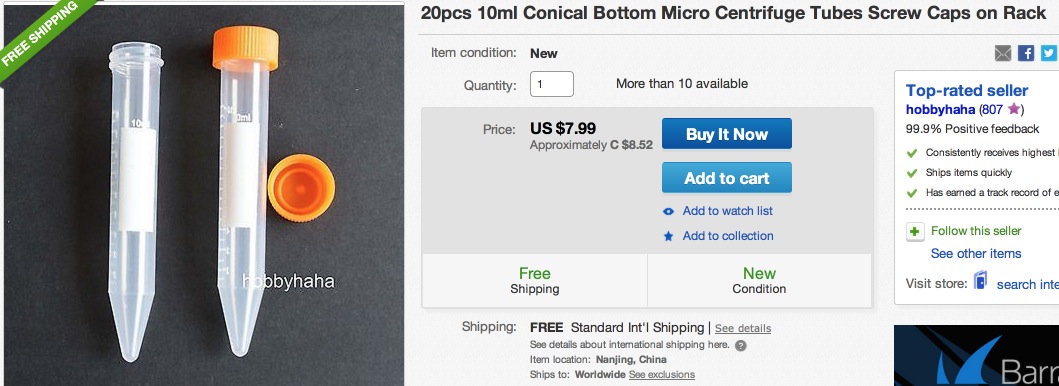
Tube Rack – if many sample tubes are to be shipped together

Sample 10 mL tubes
Labels that need to go on the box, for human or cow body fluids
Additional Packaging Requirements for Non-Infectious Clinical Samples
To ensure safe delivery of your clinical sample shipments, we provide these additional guidelines.
Liquid Clinical Sample Marking Requirements
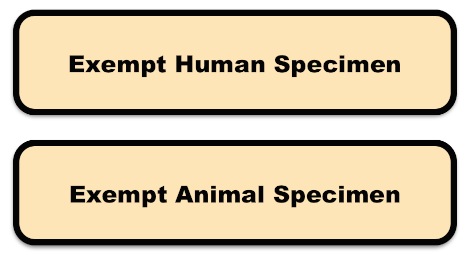
Include a marking on the package that properly identifies the shipment as “Exempt Human Specimen” or “Exempt Animal Specimen” as appropriate to comply with current IATA and ICAO regulations. If you prefer, package markings may be in the form of a label.
Dried Samples
While non-infectious samples of dried blood, tissue, saliva and hair are not dangerous goods and are not required to meet dangerous goods regulations, they do require special packaging that meets FedEx Express guidelines. Enclose dried-blood samples on absorbent pads or cards in water- tight plastic bags and ship them inside a Tyvek® or flexible plastic envelope, padded envelope, paperboard envelope or paper mailer measuring 6″ x 8″ or larger. Cushion samples on glass or plastic slides and ship them inside a sturdy outer container.

Clinical Specimen Pack front

Fedex Clinical Pack, back
Redesigned FedEx® Clinical Pak
For your convenience, we offer the FedEx Clinical Pak as an overwrap for exempt clinical-sample shipments. We recommend the FedEx Clinical Pak for use when the sturdy outer packaging of your properly packaged shipment is smaller than 7″ x 4″ x 2″ (minimum acceptable size).
To help ensure the timely delivery of your shipment and the safety of shipments exposed to yours, the FedEx Clinical Pak has been redesigned. With this redesigned overwrap, your FedEx Clinical Pak shipments stand apart from other shipments. Replace any old packaging you may have with this redesigned packaging. Since the previous FedEx Clinical Paks were made of plastics with a resin identification code of “7,” you should contact your local municipality to determine the best way to recycle any unused packaging.
The FedEx Clinical Pak can only be used to ship clinical samples. If you need an overwrap for shipments containing Biological Substance, Category B (UN 3373) materials, use the FedEx® UN 3373 Pak and package your shipments
Packaging Restrictions
- Foam boxes, plastic bags & paper envelopes are unacceptable outer containers.
- The FedEx® Envelope, FedEx® Tube, FedEx® Pak, FedEx® Padded Pak, FedEx UN 3373 Pak and FedEx boxes, including FedEx brown packaging offered at FedEx shipping locations, are not acceptable as outer containers for clinical samples.
- The FedEx Clinical Pak cannot be used to ship Biological Substance, Category B (UN 3373) shipments.
- The FedEx Clinical Pak should not be used to ship dried samples unless the dried samples are packaged following the requirements for liquid samples.
- Shipments marked or labeled 6.2(infectious materials) and shipments containing dry ice cannot be shipped in a FedEx Clinical Pak.
- If you have questions about whether your shipments require a biohazard label, consult the Occupational Safety and Health Administration (OSHA) for the applicable regulations.
NOTE: Never place liquid clinical samples in a FedEx Express® Drop Box. Select FedEx Office and FedEx World Service Center® locations accept FedEx Clinical Pak shipments that are not classified as Biological Substance, Category B (UN 3373). Call 1.800.GoFedEx 1.800.463.3339 to be directed to a FedEx location equipped to handle these shipments.
FedEx Package Evaluation Services
We offer free package evaluation services, and we encourage you to submit a sample of your clinical sample packaging for evaluation.
Clinical Packaging Evaluation Request Guidelines
Follow these steps for submitting your packaging for evaluation. An active FedEx account number is required. You and your FedEx account executive should expect evaluation results via e-mail in approximately 5 to 7 business days from FedEx Packaging Services’ receipt of your packaging.
- Obtain a FedEx Packaging Test Application, or contact FedEx Packaging Services at packagingservices@fedex.com or 1.800.633.7019.
- Complete and sign your application, referencing the name of your FedEx account executive on the form.
- Prepare a sample package including all the components in the exact configuration you intend to ship. Do not include any specimen(s). Indicate “Non-Hazardous Content” on the samples and on the sample outer box.
- Place your completed application, any pertinent product documentation and your sample clinical package labeled “Evaluate This Package” along with any necessary cushioning material in a sturdy outer container marked “Overpacked/Clinical Sample Inside.”
- Send your shipment to the address indicated on the FedEx Packaging Test Application.
Contacts and Resources
How to Pack guidelines at fedex.com/packaging.
1.800.GoFedEx 1.800.463.3339; press “81” or say “dangerous goods.”
NOTICE:
FedEx Express will refuse to accept packages that do not meet FedEx Express, government, or IATA and ICAO requirements. This brochure is in no way intended to replace requirements mandated by 49CFR and IATA. This is for informational purposes only.

Cost of shipment
The best we can guess, priority Export cost to St. Louis, Missouri for a package weighing up to 1 Lb is between $65.00 to $66.00, according to above Export Rates, page 35 dated 1st Jan 2014. If this is true, it raises the cost of shipment substantially, and it may be worthwhile to combine a few samples together, to bring average cost down.
We are planning to use bulk packaging to test urine from homeless and poor people from one location in one shot, perhaps from Vancouver or lower mainlands area. Those who are in contact with food bank services or social workers connected with the homeless and disadvantaged people, and wish to assist in this effort, please contact me.
We shall update the information as we get to know more, including setting up or locating more labs that will test Glyphosate using economical means for the public. Please stay tuned or contact me at tony.mitra@gmail.com for info.


 For the purposes of this guide, clinical samples are generally defined as non-infectious human or animal materials including, but not limited to, excreta, secreta, tissue and tissue fluids, blood and FDA-approved pharmaceuticals that are blood products.
For the purposes of this guide, clinical samples are generally defined as non-infectious human or animal materials including, but not limited to, excreta, secreta, tissue and tissue fluids, blood and FDA-approved pharmaceuticals that are blood products.








